
RECOMMENDATIONS
for Application of Autonomous Electrostimulator
of Gastrointestinal Tract and Mucous Membranes
(Electronic Normalizer)

RECOMMENDATIONS
for Application of Autonomous Electrostimulator
of Gastrointestinal Tract and Mucous Membranes
(Electronic Normalizer)
The Autonomous Electrostimulator of Gastrointestinal Tract and Mucous Membranes (Electronic Normalizer) was permitted for over-the-counter medical applications by the USSR Ministry of Public Health, the application instruction was approved on November 21, 1991 by the Russian Federal Ministry of Public Health; it is produced by Ecomed Research and Production Association.
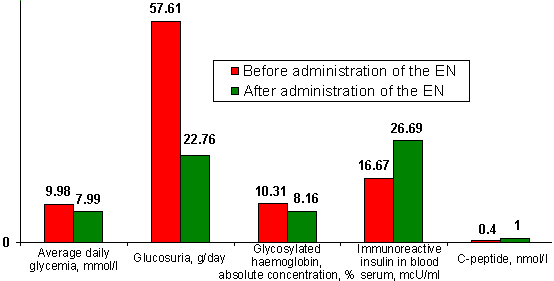
| Figure 1. | Parameters of changes in condition of carbohydrate metabolism by example of diabetes mellitus II patients treated with capsules of the Autonomous Electrostimulator of Gastrointestinal Tract and Mucous Membranes (Electronic Normalizer) (given condition on the 30-th day after single administration of one capsule of the Electronic Normalizer). |
At the same time, researchers observed a reliable decrease in total cholesterol concentration in blood serum (by 1.9 times), cholesterol of low-density lipoproteins (by 1.7 times) with a simultaneous increase in the concentration of high-density lipoproteins (by 1.8 times) [16]. In addition, the patients of diabetes mellitus II showed an increase in the initially reduced concentration of free thyroxine (by 1.4 times) and cortisole (by 1.5 times), as well as sex hormones in blood serum, which, however, does not exceed their highest values observed for virtually healthy persons [1].
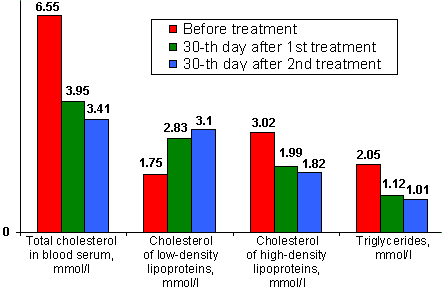
| Figure 2. | Parameters of changes in condition of lipid metabolism by example of diabetes mellitus I patients treated with capsules of the Autonomous Electrostimulator of Gastrointestinal Tract and Mucous Membranes (Electronic Normalizer). |
Application of the Electronic Normalizer in the extreme conditions (Metelitsa group expedition in Antarctica) results in a sharp improvement in the emotional status and necessary correction of hormonal status adequately to the body existence conditions [2].
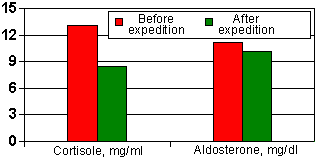
| Figure 3. | Change in parameters of adrenal hormones after administration of the Autonomous Electrostimulator of Gastrointestinal Tract and Mucous Membranes (Electronic Normalizer). |
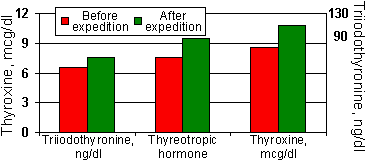
| Figure 4. | Change in the parameters of thyroid and pituitary hormones after administration of the Autonomous Electrostimulator of Gastrointestinal Tract and Mucous Membranes (Electronic Normalizer). |
The liquidators of the accident in Chernobyl Nuclear Power Station, who suffered from ischemic heart disease and hypertension, were treated with the Electronic Normalizer and showed a decrease in the parameters of lipid fraction of blood serum with a simultaneous increase in the antiteratogenic fraction of high-density lipoproteins. At the same time, the lipid-reducing effect of just a single administration of the Electronic Normalizer lasts for thirty days that cannot be virtually provided with any drugs in connection with decreased organism adaptation capacities in this category of patients [3].
In accordance with the research results, the Electronic Normalizer is recommended for application to the complex therapy of patients suffering from diabetes mellitus I and II and ischemic heart disease, in particular, in combination with expressed disorders in lipid metabolism, atherosclerotic complications, obesity, constipation.
For the purposes of electrostimulation of gastrointestinal tract, the effect of the Electronic Normalizer was studied in the group of complicated pathologies of gastrointestinal tract, namely, chronic constipation. These results showed that this kind of electrostimulation affects actively the nervous and muscular systems of the gastrointestinal tract, recovers the motor and evacuator functions, promotes improvement of self-feeling, improves working capacity and normalises psychoemotional status of patients. The positive effect was achieved for 89.4 per cent of patients with cologenic, proctogenic and combined constipation complicated with chronic diseases of other organs and systems [4] that is significantly more effective as compared to drug and multifactor therapy accepted in the world practice [7, 8].
The tests carried out in gastroenterology, therapy, as well as planned and urgent surgery showed a high efficiency of this method in treatment of diseases of gastrointestinal tract [5, 6, 7, 8]. This enabled to recommend application of the Electronic Normalizer in post-operative period for rehabilitation of dynamic passability of intestines, decrease in intoxication, strengthening of regional circulation and metabolic and trophic processes in gastrointestinal tract that promotes rehabilitation of normal organism functioning and accelerates recovery of post-operative injuries.
The patients suffering from immune deficiency and, in particular, bronchial asthma were treated with the Electronic Normalizer and showed a significant improvement in condition that enabled 30 per cent of these patients to cancel partially administration of broncholytics or antiphlogistics. All the patients showed a decrease in bronchial hyperreactivity with respect to non-specific external factors that is accounted for by a change in affinity and / or number of acetyl choline receptors to epithelia of bronchi and effector cells. Application of the Electronic Normalizer normalises the changed reactivity of air ducts. In addition, the patients show a reliable decrease in the concentration of immunoglobulin E (by 1.9 to 4 times) with a simultaneous increase in the concentration of immunoglobulin A (by 1.2 times) [9, 10].
Taking into account the normalising effect of the Electronic Normalizer on the parameters of immune system, it is expedient to recommend its application for the purposes of prevention of viral and other respiratory diseases in spring and summer periods.
In addition, administration of the Electronic Normalizer is indicated to patients suffering from bronchial asthma (in the phase of remission), allergy, expressed eosinophilia, as well as to virtually healthy persons in the period of increased physical and mental loading and stress overloading.
Application of the Electronic Normalizer promotes an improvement in the subjective condition and quality of night sleep, normalises psychoemotional status and activates psychomotor reactions on the background of some increase in the efficiency of tissular respiration, physiological optimisation of immune and haematological parameters, motor and evacuator functions of gallbladder [11].
At the same time, the efficiency of the Electronic Normalizer was noted with respect to stabilisation of the affective emotional sphere and increase in male potency in the case of complicated somatic background [14]. An increase in libido and recovery of morning erection was observed also for patients suffering from diabetes mellitus and obesity [12]. The comprehensive effect of the Electronic Normalizer enables to produce a high therapeutical result in the case of chronic prostatitis and prostatodynia. In this case, application of capsules may be either peroral or rectal (according to a certain scheme) [13].
In addition, a special interest is represented by the ability of the Electronic Normalizer to affect the parameters of the reproductive function determining the parameters of duration and quality of life [1, 3, 17].
The absence of side effects and harmful influence on human organism enables to recommend the Electronic Normalizer for wide application to medical practice not only in stationary but also in ambulatory and home conditions, including virtually healthy persons for prevention of gastroenterological, proctological, endocrine, psychoneurological and other disorders and diseases, for activation of circulation and secretion functions of endocrine glands, stimulation of muscular tone, receptor system and ducts of central and peripheral nervous system, as well as to increase the organism resistance to any kinds of infection, to normalise metabolic processes and to correct weight, to prevent osteochondrosis [15] and atherosclerosis [18].
In order to produce the highest prolonged effect in preventive and therapeutical applications, according to the results of completed set of investigations, it is recommended to take two capsules with the interval of 2 to 4 weeks for the primary administration. In complicated cases, administration is recommended under supervision of physician up to five times a year with the interval of 2 to 3 months.
The methods of therapy and correction of the organism parameters, as well as the constructive features of the Electronic Normalizer are protected by the following Patents of Russian Federation: No. 1632 dated December 28, 1993 "Electrostimulator", No. 2071368 "Electronic Normalizer", No. 2071367 "Method Increasing Organism Resistance to Tumours", No. 2071379 "Method Correcting Lipid Metabolism", No. 2081636 "Method Correcting Immunity Parameters", No. 2071637 "Method Affecting Psychiatric and Psychoneurological Capacities of Organism", No. 2081635 "Method Recovering Working Capacity", and International Patent No. PCT/RU96/00023 "Electronic Normalizer" (16 countries).
ATTENTION! The test protocols and results aforementioned in these Recommendations refer only to the Autonomous Electrostimulator of Gastrointestinal Tract and Mucous Membranes (Electronic Normalizer) produced by Ecomed Research and Production Association (TU 9444-014-11555014, diagram MASI 943115.001). Any references to the said protocols and results with respect to similar articles of other producers ARE ILLEGAL AND PROHIBITED!
The genuine articles are metal capsules with plastic insert with laser mark "Electronic Normalizer, Ecomed Research and Production Association, Patent 207136, trademark, with identification number (9 characters) and asterisk on the case. The pack is marked with band code 46055323.
| Doctor of Medical Sciences, Director of the Institute of Diabetes, I. M. Sechenov Moscow Medical Academy, Professor |
|
| /signature/ | M. I. Balabolkin |
| Honoured Scientist of Russian Federation, Deputy Director for Research, State Research Centre of Coloproctology, Russian Federal Ministry of Public Health, Professor |
|
| /signature/ | G. A. Pokrovski |
| Doctor of Medical Sciences, Director of the Moscow Research Institute of Psychiatry, Russian Federal Ministry of Public Health, Professor |
|
| /signature/ | V. N. Krasnov |
| Director of the State Research Institute of Experimental Medicine and Physical Therapy, Russian Federal Ministry of Defence, Major-General of Medical Service, Doctor of Medical Sciences |
|
| /signature/ | E. G. Zhilyaev |
| Doctor of Medical Sciences, Academician of the Russian Academy of Medical and Technical Sciences, State Prize Winner, Director of the Medical Department, Binar Research Institute, Russian Academy of Medical and Technical Sciences, Professor |
|
| /signature/ | O. A. Mashkov |